| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 5223010 | 1383474 | 2011 | 5 صفحه PDF | دانلود رایگان |
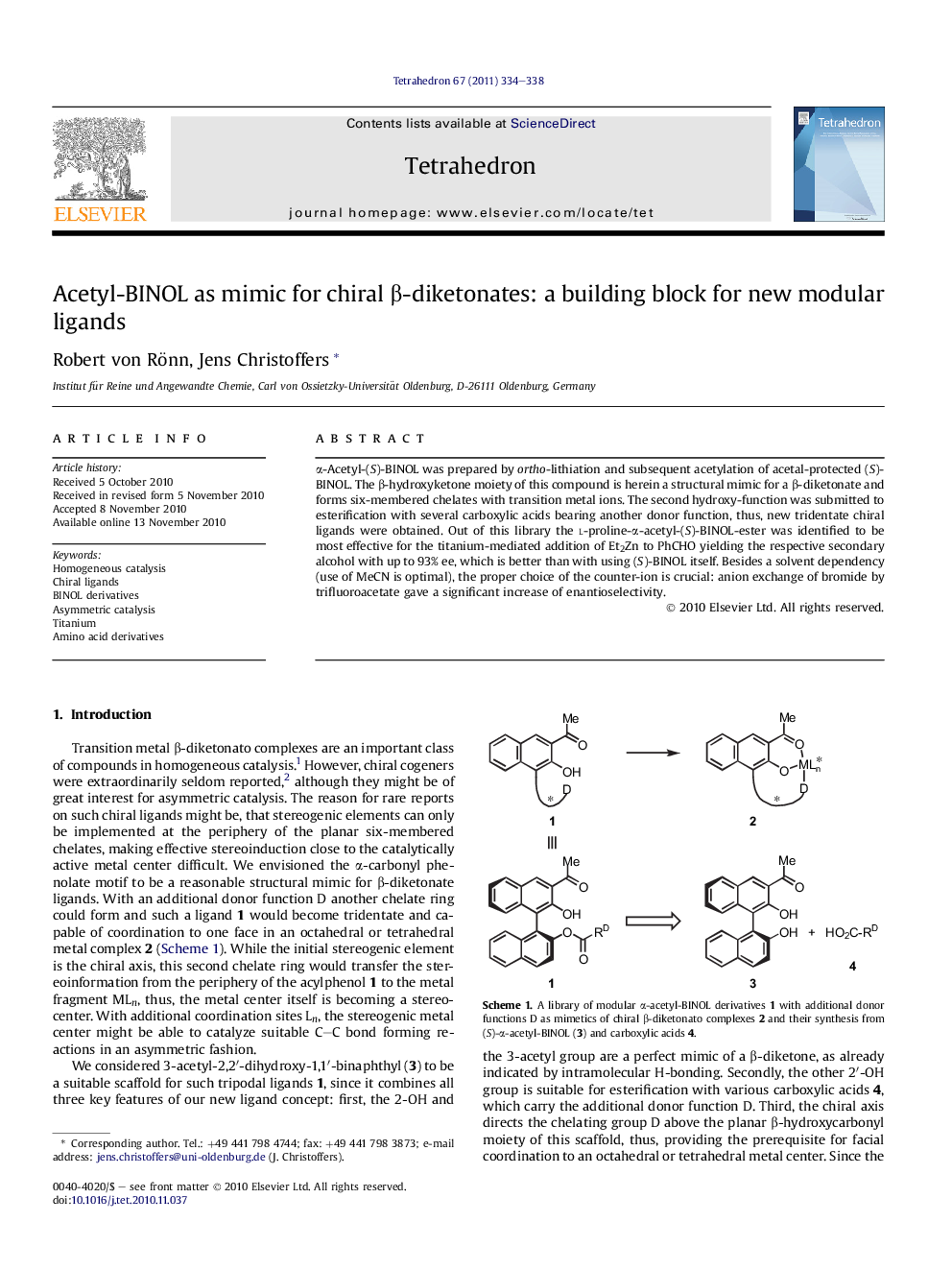
α-Acetyl-(S)-BINOL was prepared by ortho-lithiation and subsequent acetylation of acetal-protected (S)-BINOL. The β-hydroxyketone moiety of this compound is herein a structural mimic for a β-diketonate and forms six-membered chelates with transition metal ions. The second hydroxy-function was submitted to esterification with several carboxylic acids bearing another donor function, thus, new tridentate chiral ligands were obtained. Out of this library the l-proline-α-acetyl-(S)-BINOL-ester was identified to be most effective for the titanium-mediated addition of Et2Zn to PhCHO yielding the respective secondary alcohol with up to 93% ee, which is better than with using (S)-BINOL itself. Besides a solvent dependency (use of MeCN is optimal), the proper choice of the counter-ion is crucial: anion exchange of bromide by trifluoroacetate gave a significant increase of enantioselectivity.
Journal: Tetrahedron - Volume 67, Issue 2, 14 January 2011, Pages 334-338