| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 5227525 | 1383608 | 2007 | 7 صفحه PDF | دانلود رایگان |
عنوان انگلیسی مقاله ISI
Prolinamides derived from aminophenols as organocatalysts for asymmetric direct aldol reactions
دانلود مقاله + سفارش ترجمه
دانلود مقاله ISI انگلیسی
رایگان برای ایرانیان
کلمات کلیدی
موضوعات مرتبط
مهندسی و علوم پایه
شیمی
شیمی آلی
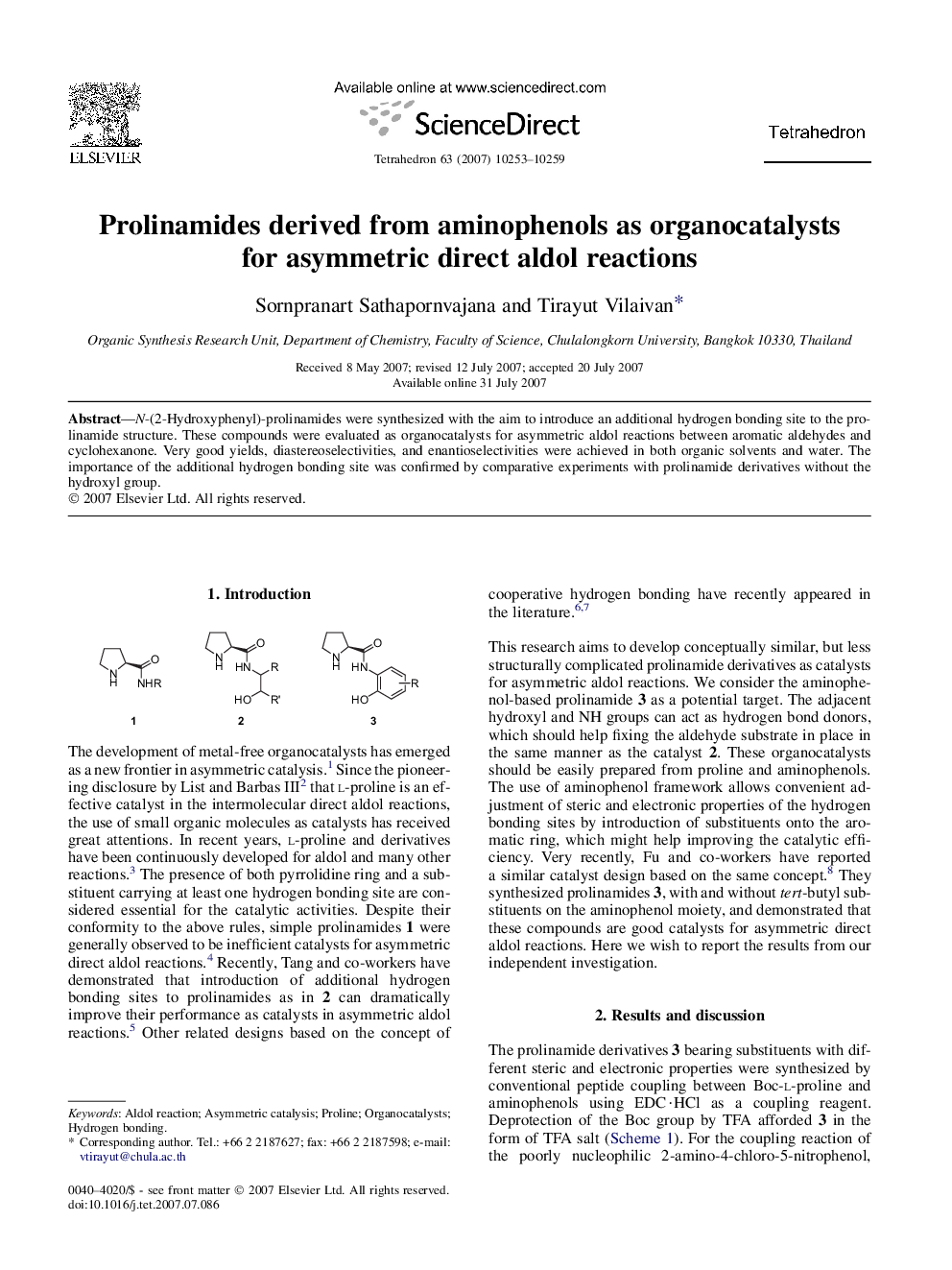
پیش نمایش صفحه اول مقاله
چکیده انگلیسی
N-(2-Hydroxyphenyl)-prolinamides were synthesized with the aim to introduce an additional hydrogen bonding site to the prolinamide structure. These compounds were evaluated as organocatalysts for asymmetric aldol reactions between aromatic aldehydes and cyclohexanone. Very good yields, diastereoselectivities, and enantioselectivities were achieved in both organic solvents and water. The importance of the additional hydrogen bonding site was confirmed by comparative experiments with prolinamide derivatives without the hydroxyl group.
A series of prolinamides derived from 2-aminophenols were synthesized and shown to be efficient organocatalysts for asymmetric aldol reactions between aromatic aldehydes and cyclohexanone.
ناشر
Database: Elsevier - ScienceDirect (ساینس دایرکت)
Journal: Tetrahedron - Volume 63, Issue 41, 8 October 2007, Pages 10253-10259
Journal: Tetrahedron - Volume 63, Issue 41, 8 October 2007, Pages 10253-10259
نویسندگان
Sornpranart Sathapornvajana, Tirayut Vilaivan,