| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 5747151 | 1618790 | 2017 | 8 صفحه PDF | دانلود رایگان |
- Calcined hydrotalcite HTC was tested for selenium species sorption-desorption.
- HTC sorption-desorption capacity was analysed in solid phase by EDXRF.
- Mathematical modelling allowed us to estimate selenium mobilization in environment.
- Hysteresis index and mobilization factor were calculated to estimate reversibility.
- HTC desorption capacity was explored for quantitation purposes in aquatic systems.
Selenate and selenite are considered emerging contaminants and pose a risk to living organisms. Since selenium anion species are at low concentration in aquatic environments, materials for its retention are required to enable monitoring. Herein, hydrotalcite was calcined and characterised to investigate sorption and desorption of selenite and selenate in competition with nitrate, sulfate and phosphate. Sorption experiments were carried out in batch system and desorption by sequential dilution. Selenite and selenate concentration remaining after N desorption steps was determined by mass balance. The isotherms were adjusted to the dual-mode Langmuir-Freundlich model (R2Â >Â 0.99). Maximum sorption capacity ranged from 494 to 563 meq kgâ1 for selenite and from 609 to 659 meq kgâ1 for selenate. Sulfate and phosphate ions showed greater competitive effect on the sorption of selenate and selenite, respectively. Low mobilization factors and high sorption efficiency (MF<3%; SE â 100%) indicated that calcined hydrotalcite has the wanted characteristics for retention of relevant selenium anion species in aqueous media.
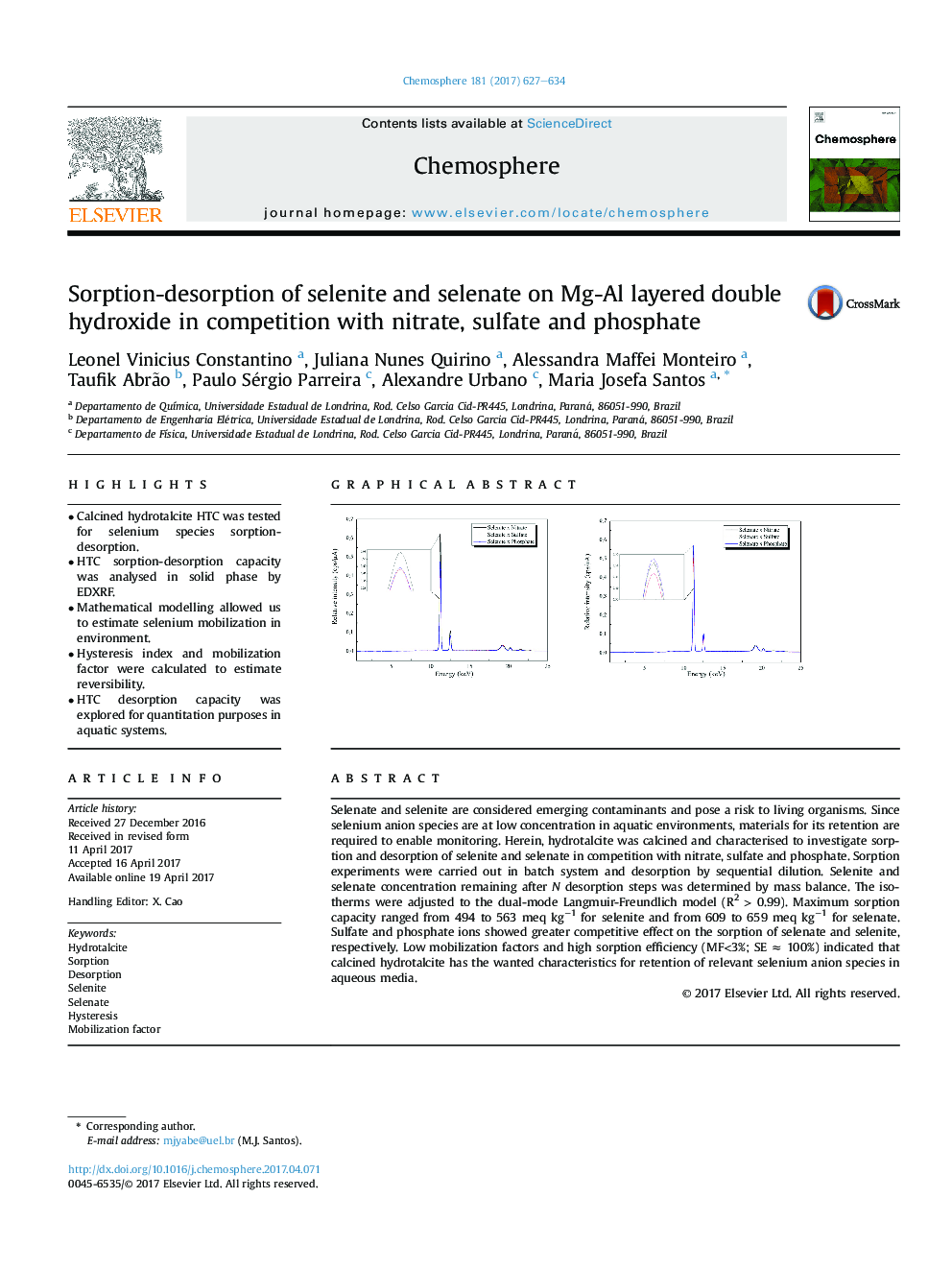
EDXRF spectra of selenite and selenate onto HTC after sorption; both from 10 meq Lâ1 initial concentration in presence of nitrate, sulfate and phosphate. In detail the overlap of selenium peaks in presence of interfering anions.167
Journal: Chemosphere - Volume 181, August 2017, Pages 627-634