| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 593676 | 1453951 | 2013 | 8 صفحه PDF | دانلود رایگان |
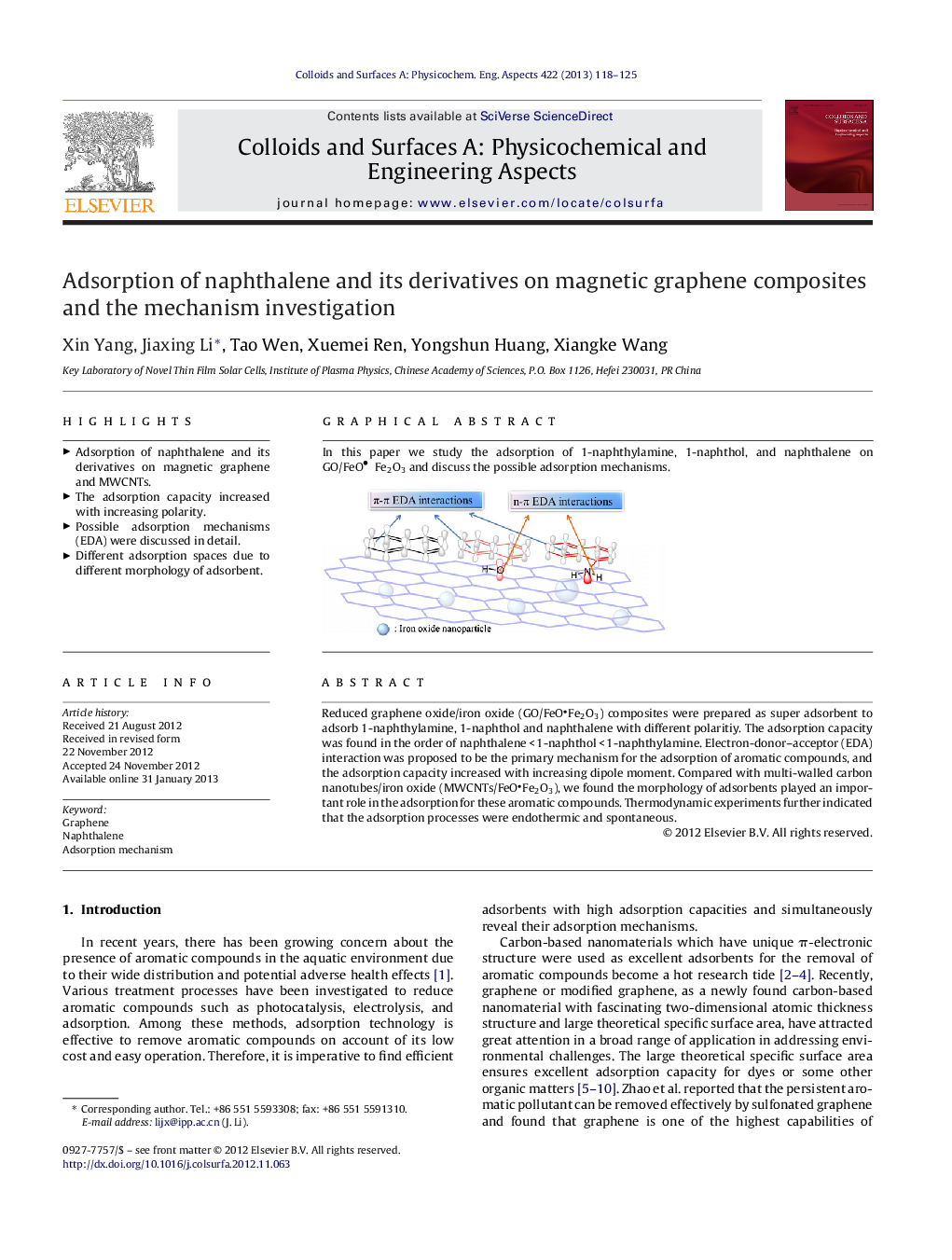
Reduced graphene oxide/iron oxide (GO/FeOFe2O3) composites were prepared as super adsorbent to adsorb 1-naphthylamine, 1-naphthol and naphthalene with different polaritiy. The adsorption capacity was found in the order of naphthalene < 1-naphthol < 1-naphthylamine. Electron-donor–acceptor (EDA) interaction was proposed to be the primary mechanism for the adsorption of aromatic compounds, and the adsorption capacity increased with increasing dipole moment. Compared with multi-walled carbon nanotubes/iron oxide (MWCNTs/FeOFe2O3), we found the morphology of adsorbents played an important role in the adsorption for these aromatic compounds. Thermodynamic experiments further indicated that the adsorption processes were endothermic and spontaneous.
In this paper we study the adsorption of 1-naphthylamine, 1-naphthol, and naphthalene on GO/FeOFe2O3 and discuss the possible adsorption mechanisms.Figure optionsDownload as PowerPoint slideHighlights
► Adsorption of naphthalene and its derivatives on magnetic graphene and MWCNTs.
► The adsorption capacity increased with increasing polarity.
► Possible adsorption mechanisms (EDA) were discussed in detail.
► Different adsorption spaces due to different morphology of adsorbent.
Journal: Colloids and Surfaces A: Physicochemical and Engineering Aspects - Volume 422, 5 April 2013, Pages 118–125