| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 593700 | 1453953 | 2013 | 7 صفحه PDF | دانلود رایگان |
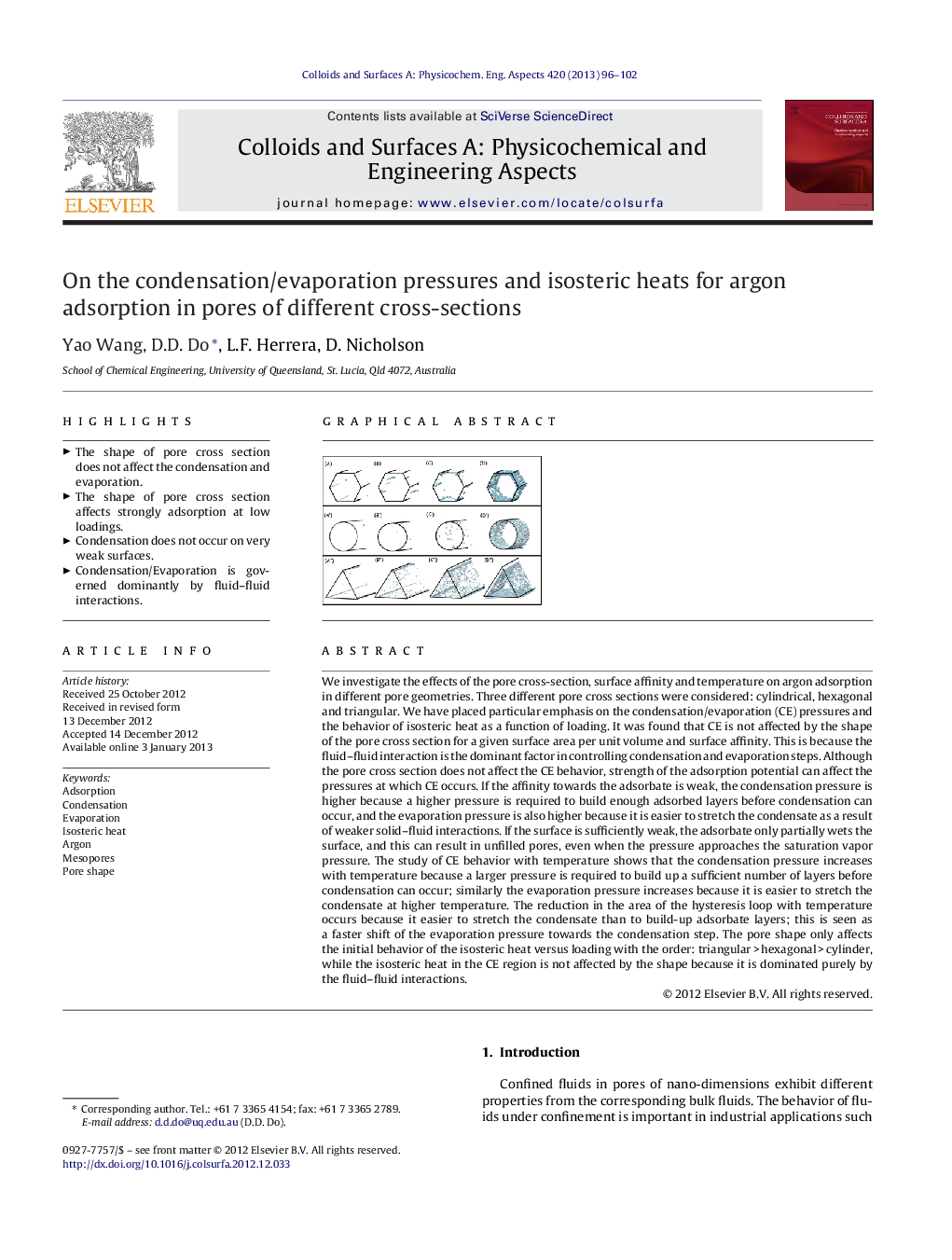
We investigate the effects of the pore cross-section, surface affinity and temperature on argon adsorption in different pore geometries. Three different pore cross sections were considered: cylindrical, hexagonal and triangular. We have placed particular emphasis on the condensation/evaporation (CE) pressures and the behavior of isosteric heat as a function of loading. It was found that CE is not affected by the shape of the pore cross section for a given surface area per unit volume and surface affinity. This is because the fluid–fluid interaction is the dominant factor in controlling condensation and evaporation steps. Although the pore cross section does not affect the CE behavior, strength of the adsorption potential can affect the pressures at which CE occurs. If the affinity towards the adsorbate is weak, the condensation pressure is higher because a higher pressure is required to build enough adsorbed layers before condensation can occur, and the evaporation pressure is also higher because it is easier to stretch the condensate as a result of weaker solid–fluid interactions. If the surface is sufficiently weak, the adsorbate only partially wets the surface, and this can result in unfilled pores, even when the pressure approaches the saturation vapor pressure. The study of CE behavior with temperature shows that the condensation pressure increases with temperature because a larger pressure is required to build up a sufficient number of layers before condensation can occur; similarly the evaporation pressure increases because it is easier to stretch the condensate at higher temperature. The reduction in the area of the hysteresis loop with temperature occurs because it easier to stretch the condensate than to build-up adsorbate layers; this is seen as a faster shift of the evaporation pressure towards the condensation step. The pore shape only affects the initial behavior of the isosteric heat versus loading with the order: triangular > hexagonal > cylinder, while the isosteric heat in the CE region is not affected by the shape because it is dominated purely by the fluid–fluid interactions.
Figure optionsDownload as PowerPoint slideHighlights
► The shape of pore cross section does not affect the condensation and evaporation.
► The shape of pore cross section affects strongly adsorption at low loadings.
► Condensation does not occur on very weak surfaces.
► Condensation/Evaporation is governed dominantly by fluid–fluid interactions.
Journal: Colloids and Surfaces A: Physicochemical and Engineering Aspects - Volume 420, 5 March 2013, Pages 96–102