| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 593779 | 1453954 | 2013 | 8 صفحه PDF | دانلود رایگان |
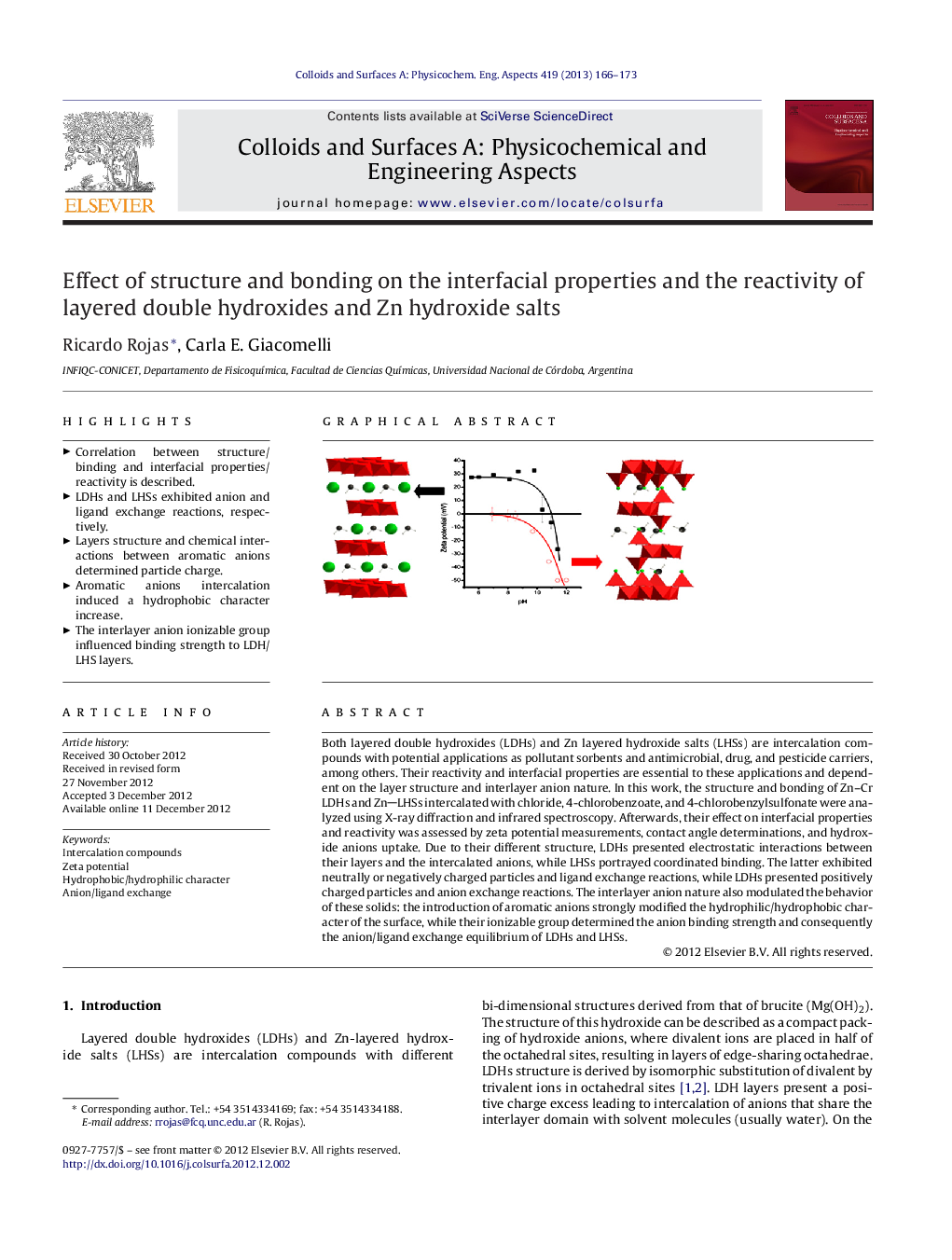
Both layered double hydroxides (LDHs) and Zn layered hydroxide salts (LHSs) are intercalation compounds with potential applications as pollutant sorbents and antimicrobial, drug, and pesticide carriers, among others. Their reactivity and interfacial properties are essential to these applications and dependent on the layer structure and interlayer anion nature. In this work, the structure and bonding of Zn–Cr LDHs and ZnLHSs intercalated with chloride, 4-chlorobenzoate, and 4-chlorobenzylsulfonate were analyzed using X-ray diffraction and infrared spectroscopy. Afterwards, their effect on interfacial properties and reactivity was assessed by zeta potential measurements, contact angle determinations, and hydroxide anions uptake. Due to their different structure, LDHs presented electrostatic interactions between their layers and the intercalated anions, while LHSs portrayed coordinated binding. The latter exhibited neutrally or negatively charged particles and ligand exchange reactions, while LDHs presented positively charged particles and anion exchange reactions. The interlayer anion nature also modulated the behavior of these solids: the introduction of aromatic anions strongly modified the hydrophilic/hydrophobic character of the surface, while their ionizable group determined the anion binding strength and consequently the anion/ligand exchange equilibrium of LDHs and LHSs.
Figure optionsDownload as PowerPoint slideHighlights
► Correlation between structure/binding and interfacial properties/reactivity is described.
► LDHs and LHSs exhibited anion and ligand exchange reactions, respectively.
► Layers structure and chemical interactions between aromatic anions determined particle charge.
► Aromatic anions intercalation induced a hydrophobic character increase.
► The interlayer anion ionizable group influenced binding strength to LDH/LHS layers.
Journal: Colloids and Surfaces A: Physicochemical and Engineering Aspects - Volume 419, 20 February 2013, Pages 166–173