| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 594065 | 1453964 | 2012 | 9 صفحه PDF | دانلود رایگان |
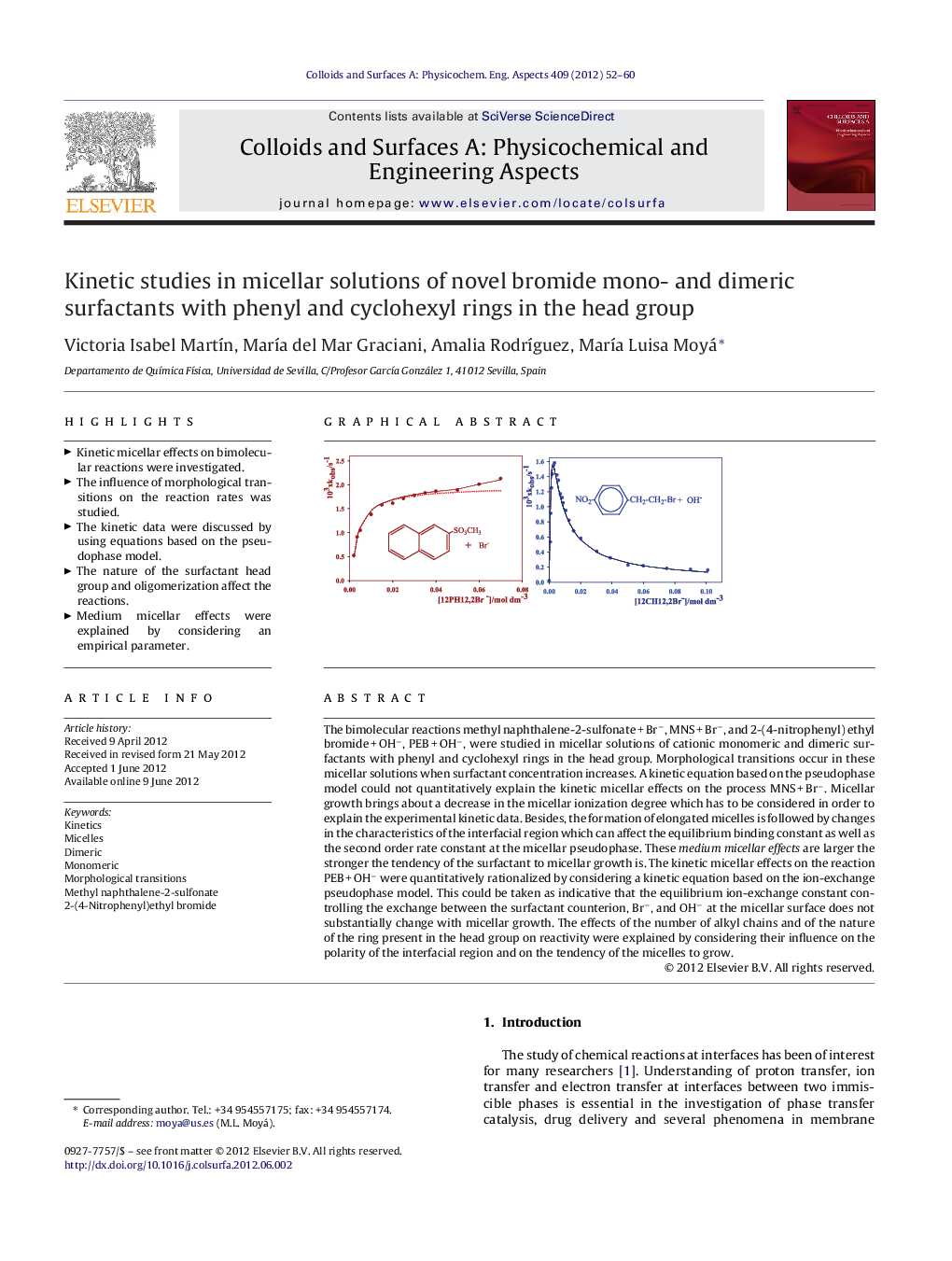
The bimolecular reactions methyl naphthalene-2-sulfonate + Br−, MNS + Br−, and 2-(4-nitrophenyl) ethyl bromide + OH−, PEB + OH−, were studied in micellar solutions of cationic monomeric and dimeric surfactants with phenyl and cyclohexyl rings in the head group. Morphological transitions occur in these micellar solutions when surfactant concentration increases. A kinetic equation based on the pseudophase model could not quantitatively explain the kinetic micellar effects on the process MNS + Br−. Micellar growth brings about a decrease in the micellar ionization degree which has to be considered in order to explain the experimental kinetic data. Besides, the formation of elongated micelles is followed by changes in the characteristics of the interfacial region which can affect the equilibrium binding constant as well as the second order rate constant at the micellar pseudophase. These medium micellar effects are larger the stronger the tendency of the surfactant to micellar growth is. The kinetic micellar effects on the reaction PEB + OH− were quantitatively rationalized by considering a kinetic equation based on the ion-exchange pseudophase model. This could be taken as indicative that the equilibrium ion-exchange constant controlling the exchange between the surfactant counterion, Br−, and OH− at the micellar surface does not substantially change with micellar growth. The effects of the number of alkyl chains and of the nature of the ring present in the head group on reactivity were explained by considering their influence on the polarity of the interfacial region and on the tendency of the micelles to grow.
Figure optionsDownload as PowerPoint slideHighlights
► Kinetic micellar effects on bimolecular reactions were investigated.
► The influence of morphological transitions on the reaction rates was studied.
► The kinetic data were discussed by using equations based on the pseudophase model.
► The nature of the surfactant head group and oligomerization affect the reactions.
► Medium micellar effects were explained by considering an empirical parameter.
Journal: Colloids and Surfaces A: Physicochemical and Engineering Aspects - Volume 409, 5 September 2012, Pages 52–60