| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 6465511 | 1422952 | 2017 | 9 صفحه PDF | دانلود رایگان |
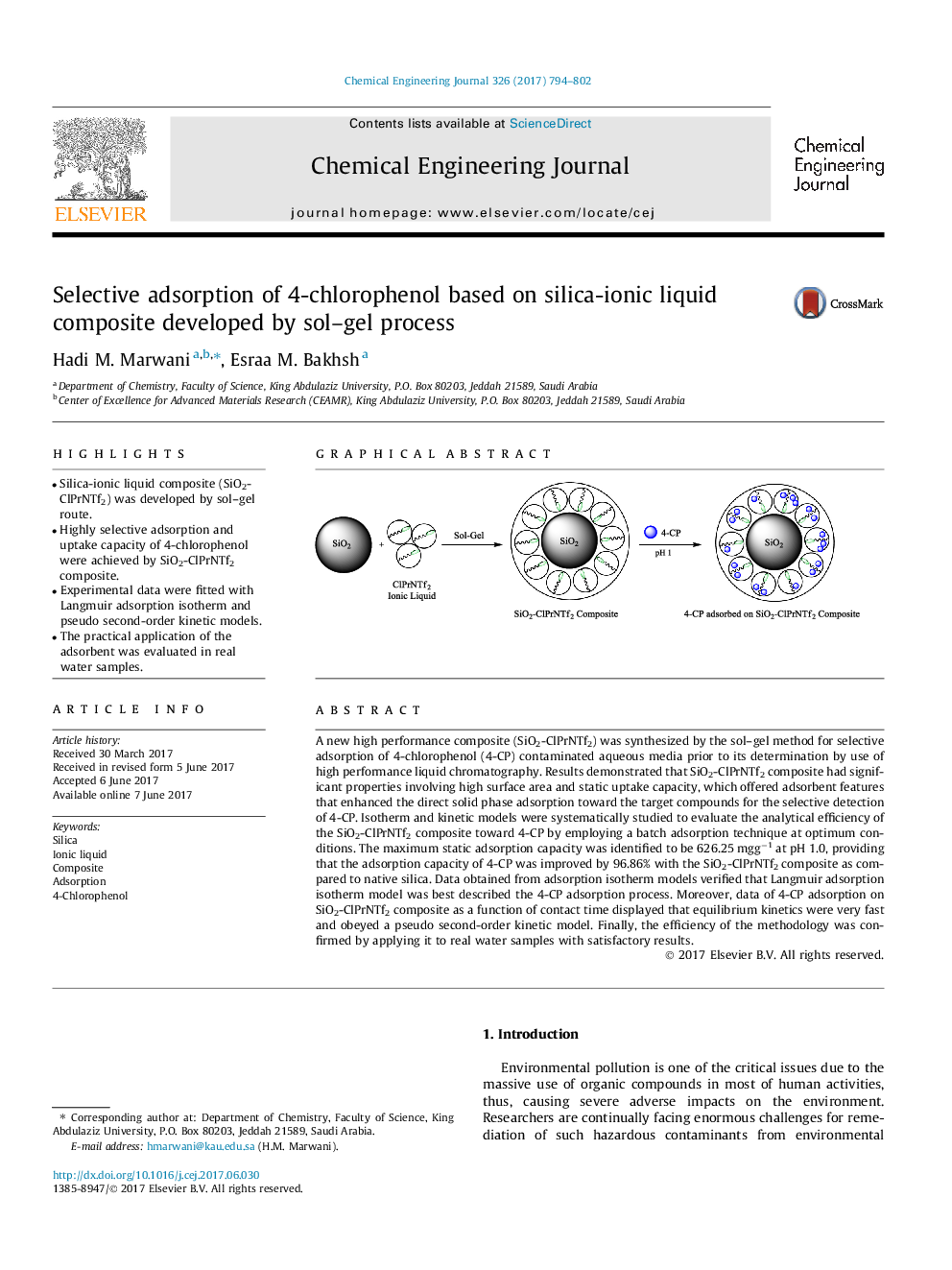
- Silica-ionic liquid composite (SiO2-ClPrNTf2) was developed by sol-gel route.
- Highly selective adsorption and uptake capacity of 4-chlorophenol were achieved by SiO2-ClPrNTf2 composite.
- Experimental data were fitted with Langmuir adsorption isotherm and pseudo second-order kinetic models.
- The practical application of the adsorbent was evaluated in real water samples.
A new high performance composite (SiO2-ClPrNTf2) was synthesized by the sol-gel method for selective adsorption of 4-chlorophenol (4-CP) contaminated aqueous media prior to its determination by use of high performance liquid chromatography. Results demonstrated that SiO2-ClPrNTf2 composite had significant properties involving high surface area and static uptake capacity, which offered adsorbent features that enhanced the direct solid phase adsorption toward the target compounds for the selective detection of 4-CP. Isotherm and kinetic models were systematically studied to evaluate the analytical efficiency of the SiO2-ClPrNTf2 composite toward 4-CP by employing a batch adsorption technique at optimum conditions. The maximum static adsorption capacity was identified to be 626.25Â mggâ1 at pH 1.0, providing that the adsorption capacity of 4-CP was improved by 96.86% with the SiO2-ClPrNTf2 composite as compared to native silica. Data obtained from adsorption isotherm models verified that Langmuir adsorption isotherm model was best described the 4-CP adsorption process. Moreover, data of 4-CP adsorption on SiO2-ClPrNTf2 composite as a function of contact time displayed that equilibrium kinetics were very fast and obeyed a pseudo second-order kinetic model. Finally, the efficiency of the methodology was confirmed by applying it to real water samples with satisfactory results.
166
Journal: Chemical Engineering Journal - Volume 326, 15 October 2017, Pages 794-802