| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1163439 | 1490955 | 2015 | 8 صفحه PDF | دانلود رایگان |
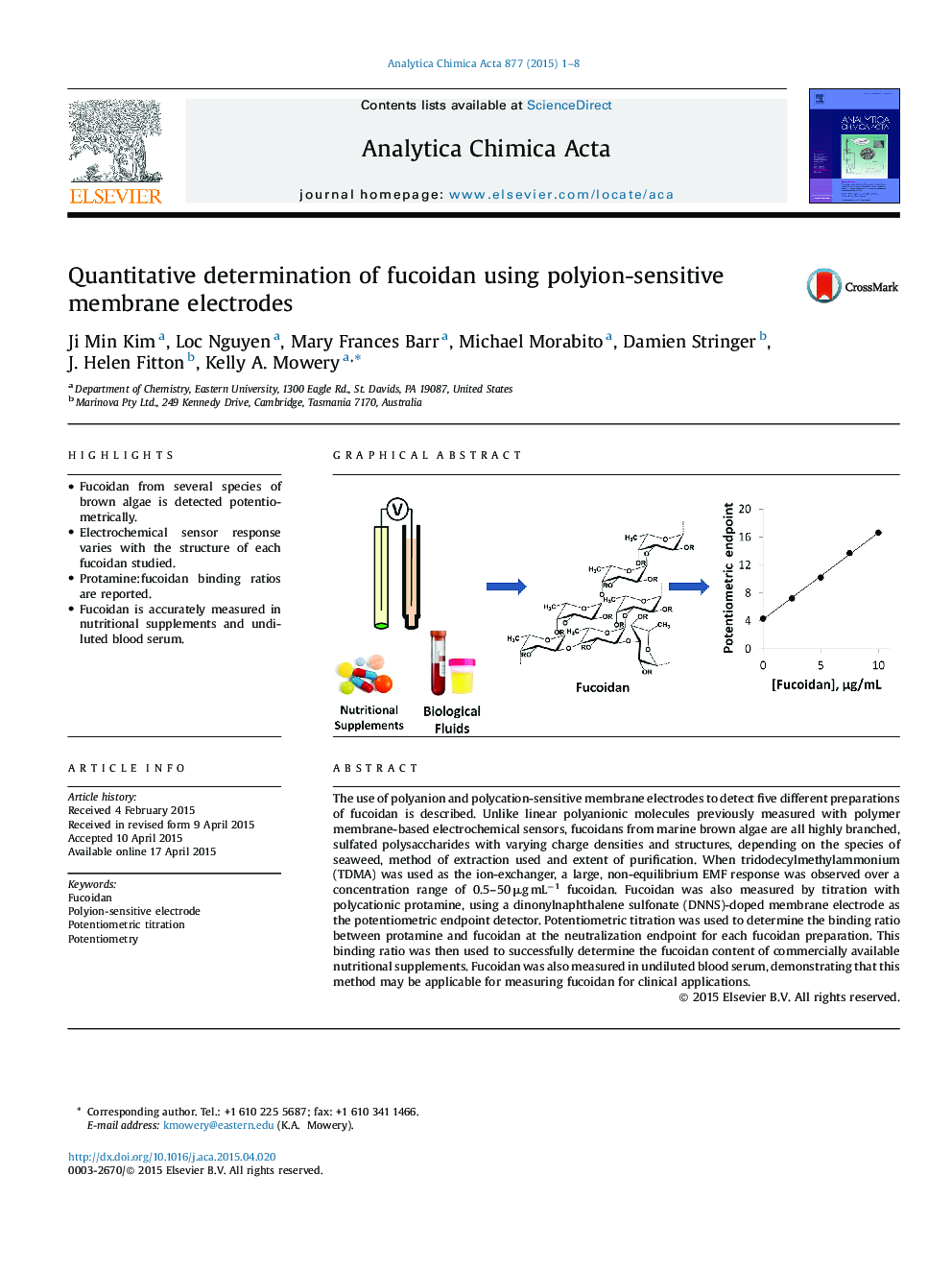
• Fucoidan from several species of brown algae is detected potentiometrically.
• Electrochemical sensor response varies with the structure of each fucoidan studied.
• Protamine:fucoidan binding ratios are reported.
• Fucoidan is accurately measured in nutritional supplements and undiluted blood serum.
The use of polyanion and polycation-sensitive membrane electrodes to detect five different preparations of fucoidan is described. Unlike linear polyanionic molecules previously measured with polymer membrane-based electrochemical sensors, fucoidans from marine brown algae are all highly branched, sulfated polysaccharides with varying charge densities and structures, depending on the species of seaweed, method of extraction used and extent of purification. When tridodecylmethylammonium (TDMA) was used as the ion-exchanger, a large, non-equilibrium EMF response was observed over a concentration range of 0.5–50 μg mL−1 fucoidan. Fucoidan was also measured by titration with polycationic protamine, using a dinonylnaphthalene sulfonate (DNNS)-doped membrane electrode as the potentiometric endpoint detector. Potentiometric titration was used to determine the binding ratio between protamine and fucoidan at the neutralization endpoint for each fucoidan preparation. This binding ratio was then used to successfully determine the fucoidan content of commercially available nutritional supplements. Fucoidan was also measured in undiluted blood serum, demonstrating that this method may be applicable for measuring fucoidan for clinical applications.
Figure optionsDownload as PowerPoint slide
Journal: Analytica Chimica Acta - Volume 877, 2 June 2015, Pages 1–8