| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1165535 | 1491065 | 2013 | 6 صفحه PDF | دانلود رایگان |
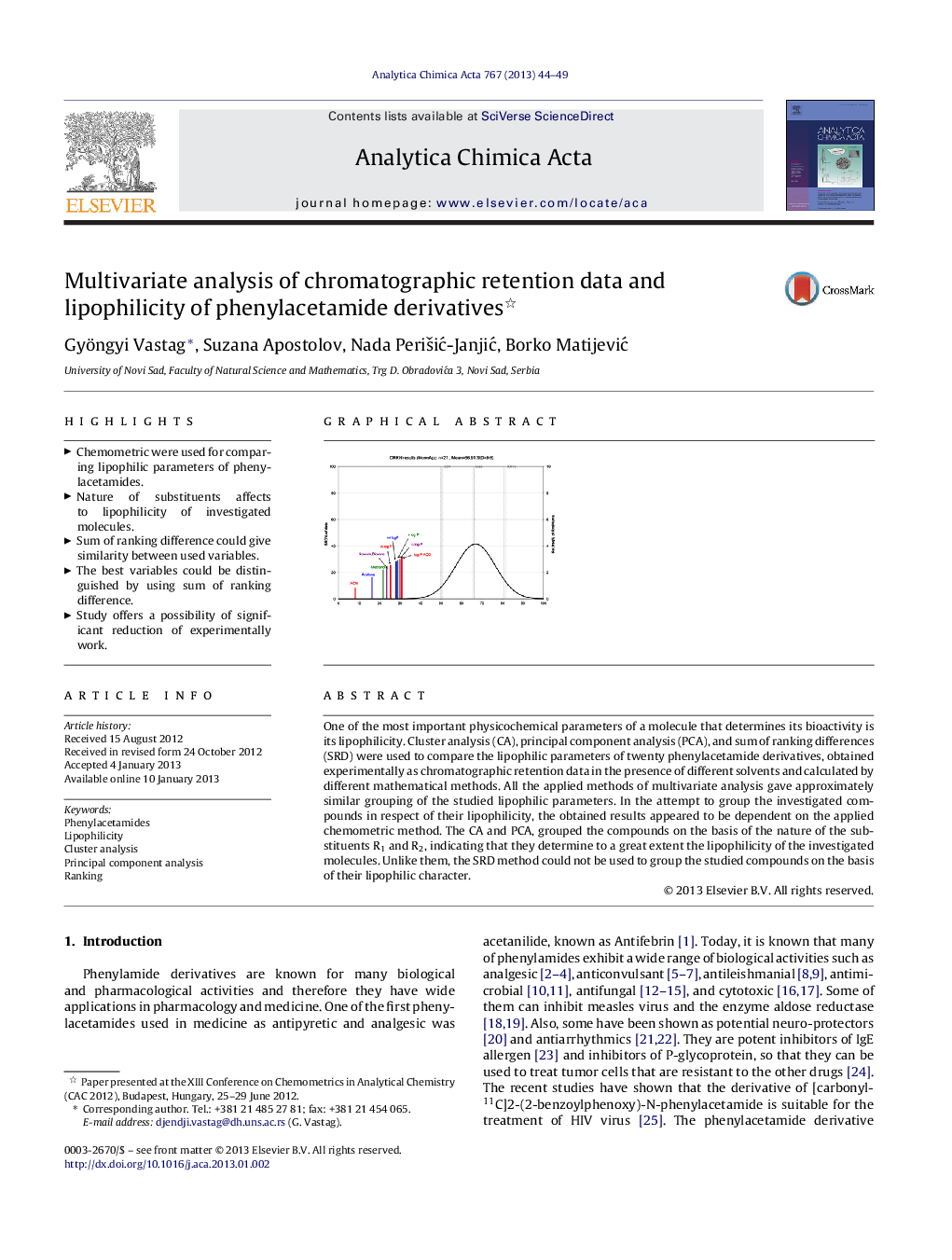
One of the most important physicochemical parameters of a molecule that determines its bioactivity is its lipophilicity. Cluster analysis (CA), principal component analysis (PCA), and sum of ranking differences (SRD) were used to compare the lipophilic parameters of twenty phenylacetamide derivatives, obtained experimentally as chromatographic retention data in the presence of different solvents and calculated by different mathematical methods. All the applied methods of multivariate analysis gave approximately similar grouping of the studied lipophilic parameters. In the attempt to group the investigated compounds in respect of their lipophilicity, the obtained results appeared to be dependent on the applied chemometric method. The CA and PCA, grouped the compounds on the basis of the nature of the substituents R1 and R2, indicating that they determine to a great extent the lipophilicity of the investigated molecules. Unlike them, the SRD method could not be used to group the studied compounds on the basis of their lipophilic character.
Figure optionsDownload as PowerPoint slideHighlights
► Chemometric were used for comparing lipophilic parameters of phenylacetamides.
► Nature of substituents affects to lipophilicity of investigated molecules.
► Sum of ranking difference could give similarity between used variables.
► The best variables could be distinguished by using sum of ranking difference.
► Study offers a possibility of significant reduction of experimentally work.
Journal: Analytica Chimica Acta - Volume 767, 12 March 2013, Pages 44–49