| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1360541 | 981439 | 2009 | 7 صفحه PDF | دانلود رایگان |
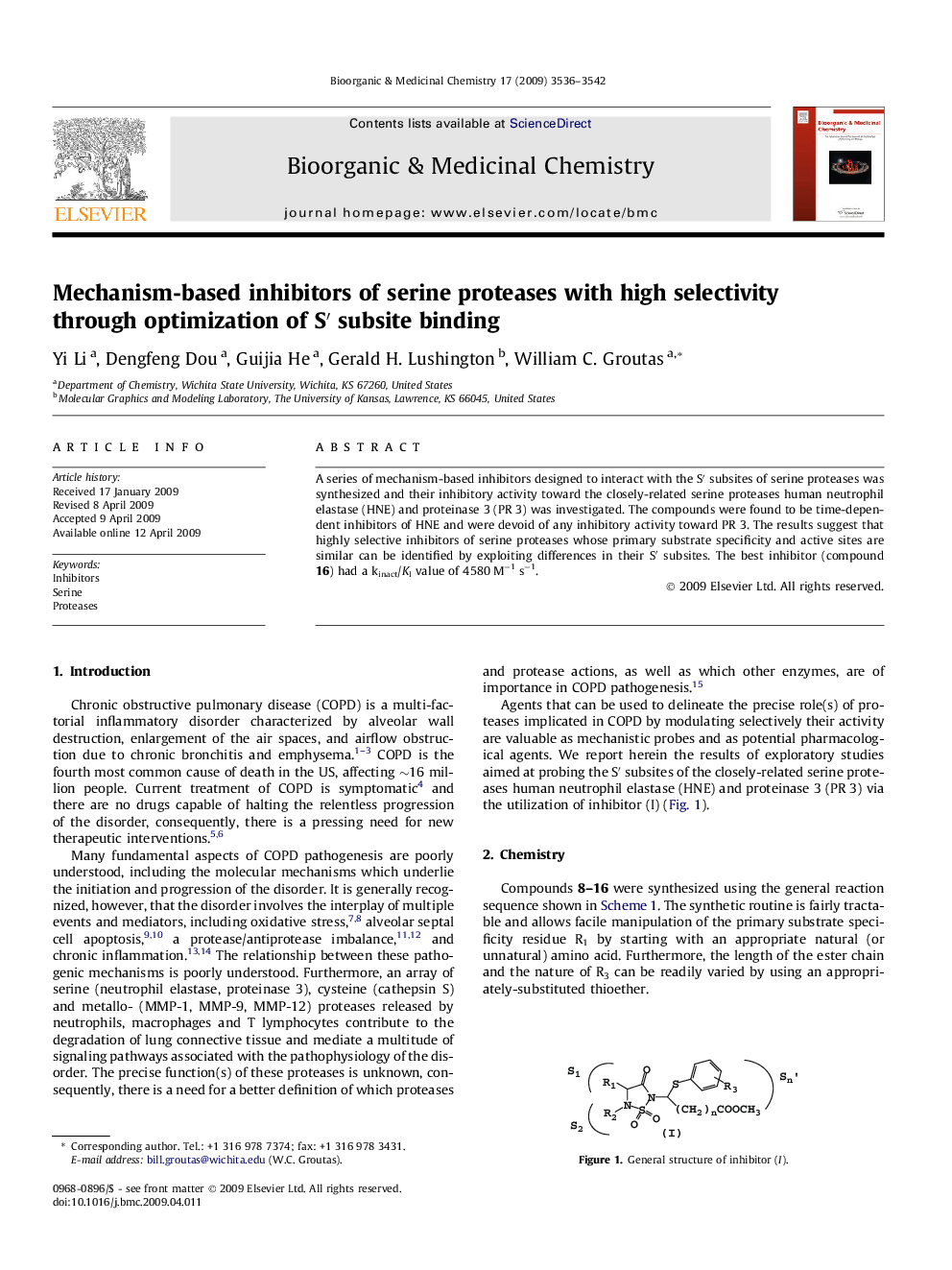
A series of mechanism-based inhibitors designed to interact with the S′ subsites of serine proteases was synthesized and their inhibitory activity toward the closely-related serine proteases human neutrophil elastase (HNE) and proteinase 3 (PR 3) was investigated. The compounds were found to be time-dependent inhibitors of HNE and were devoid of any inhibitory activity toward PR 3. The results suggest that highly selective inhibitors of serine proteases whose primary substrate specificity and active sites are similar can be identified by exploiting differences in their S′ subsites. The best inhibitor (compound 16) had a kinact/KI value of 4580 M−1 s−1.
A series of 1,2,5-thiadiazolidin-3-one 1,1-dioxide derivatives were found to be potent and selective inhibitors of human neutrophil elastase but not proteinase 3.Figure optionsDownload as PowerPoint slide
Journal: Bioorganic & Medicinal Chemistry - Volume 17, Issue 10, 15 May 2009, Pages 3536–3542