| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 4407863 | 1618823 | 2016 | 10 صفحه PDF | دانلود رایگان |
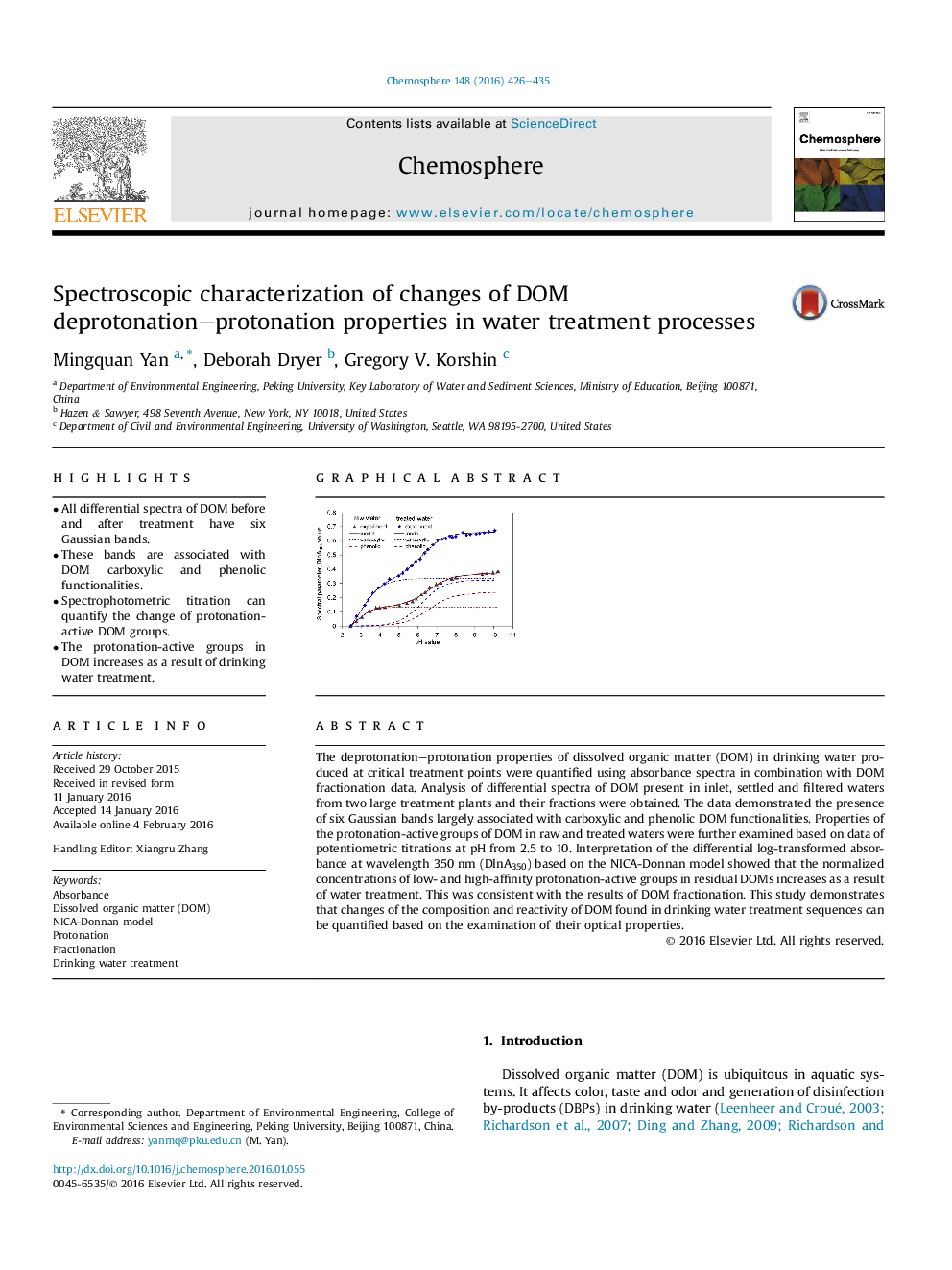
• All differential spectra of DOM before and after treatment have six Gaussian bands.
• These bands are associated with DOM carboxylic and phenolic functionalities.
• Spectrophotometric titration can quantify the change of protonation-active DOM groups.
• The protonation-active groups in DOM increases as a result of drinking water treatment.
The deprotonation–protonation properties of dissolved organic matter (DOM) in drinking water produced at critical treatment points were quantified using absorbance spectra in combination with DOM fractionation data. Analysis of differential spectra of DOM present in inlet, settled and filtered waters from two large treatment plants and their fractions were obtained. The data demonstrated the presence of six Gaussian bands largely associated with carboxylic and phenolic DOM functionalities. Properties of the protonation-active groups of DOM in raw and treated waters were further examined based on data of potentiometric titrations at pH from 2.5 to 10. Interpretation of the differential log-transformed absorbance at wavelength 350 nm (DlnA350) based on the NICA-Donnan model showed that the normalized concentrations of low- and high-affinity protonation-active groups in residual DOMs increases as a result of water treatment. This was consistent with the results of DOM fractionation. This study demonstrates that changes of the composition and reactivity of DOM found in drinking water treatment sequences can be quantified based on the examination of their optical properties.
Figure optionsDownload as PowerPoint slide
Journal: Chemosphere - Volume 148, April 2016, Pages 426–435