| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 5371165 | 1503938 | 2013 | 10 صفحه PDF | دانلود رایگان |
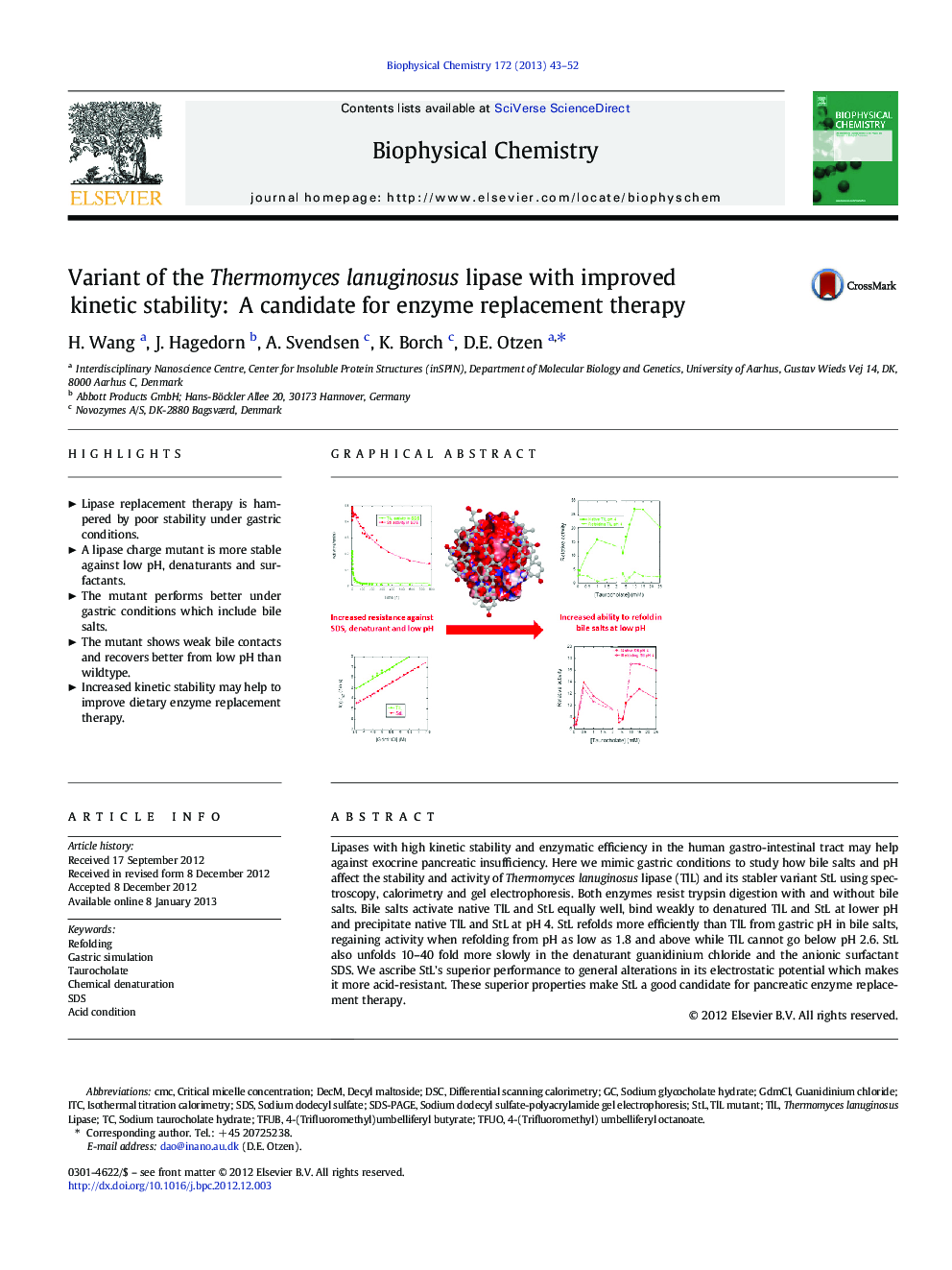
Lipases with high kinetic stability and enzymatic efficiency in the human gastro-intestinal tract may help against exocrine pancreatic insufficiency. Here we mimic gastric conditions to study how bile salts and pH affect the stability and activity of Thermomyces lanuginosus lipase (TlL) and its stabler variant StL using spectroscopy, calorimetry and gel electrophoresis. Both enzymes resist trypsin digestion with and without bile salts. Bile salts activate native TlL and StL equally well, bind weakly to denatured TlL and StL at lower pH and precipitate native TlL and StL at pH 4. StL refolds more efficiently than TlL from gastric pH in bile salts, regaining activity when refolding from pH as low as 1.8 and above while TlL cannot go below pH 2.6. StL also unfolds 10-40 fold more slowly in the denaturant guanidinium chloride and the anionic surfactant SDS. We ascribe StL's superior performance to general alterations in its electrostatic potential which makes it more acid-resistant. These superior properties make StL a good candidate for pancreatic enzyme replacement therapy.
Highlights⺠Lipase replacement therapy is hampered by poor stability under gastric conditions. ⺠A lipase charge mutant is more stable against low pH, denaturants and surfactants. ⺠The mutant performs better under gastric conditions which include bile salts. ⺠The mutant shows weak bile contacts and recovers better from low pH than wildtype. ⺠Increased kinetic stability may help to improve dietary enzyme replacement therapy.
Journal: Biophysical Chemistry - Volume 172, February 2013, Pages 43-52