| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 591906 | 1453884 | 2016 | 8 صفحه PDF | دانلود رایگان |
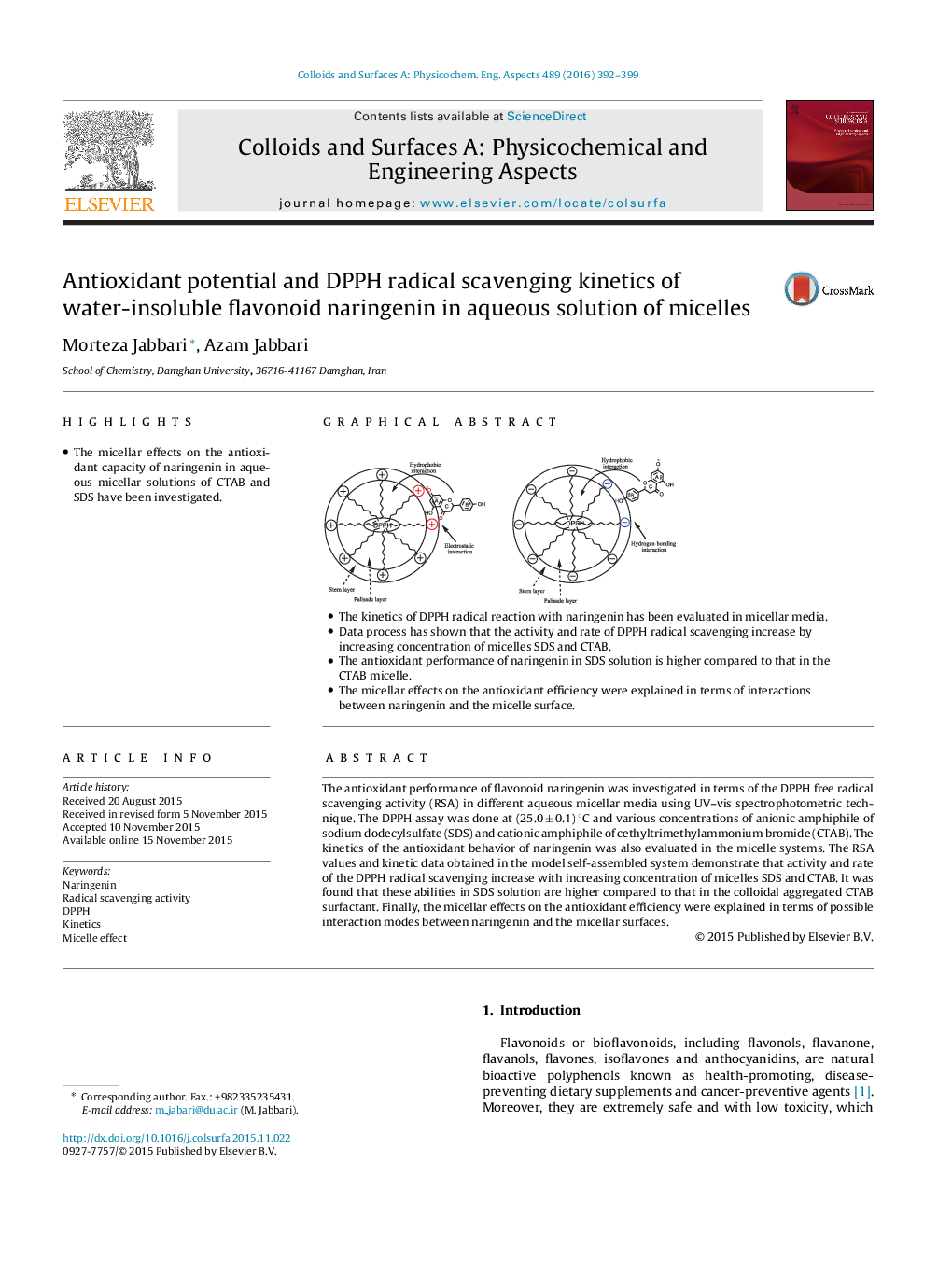
• The micellar effects on the antioxidant capacity of naringenin in aqueous micellar solutions of CTAB and SDS have been investigated.
• The kinetics of DPPH radical reaction with naringenin has been evaluated in micellar media.
• Data process has shown that the activity and rate of DPPH radical scavenging increase by increasing concentration of micelles SDS and CTAB.
• The antioxidant performance of naringenin in SDS solution is higher compared to that in the CTAB micelle.
• The micellar effects on the antioxidant efficiency were explained in terms of interactions between naringenin and the micelle surface.
The antioxidant performance of flavonoid naringenin was investigated in terms of the DPPH free radical scavenging activity (RSA) in different aqueous micellar media using UV–vis spectrophotometric technique. The DPPH assay was done at (25.0 ± 0.1) °C and various concentrations of anionic amphiphile of sodium dodecylsulfate (SDS) and cationic amphiphile of cethyltrimethylammonium bromide (CTAB). The kinetics of the antioxidant behavior of naringenin was also evaluated in the micelle systems. The RSA values and kinetic data obtained in the model self-assembled system demonstrate that activity and rate of the DPPH radical scavenging increase with increasing concentration of micelles SDS and CTAB. It was found that these abilities in SDS solution are higher compared to that in the colloidal aggregated CTAB surfactant. Finally, the micellar effects on the antioxidant efficiency were explained in terms of possible interaction modes between naringenin and the micellar surfaces.
Figure optionsDownload as PowerPoint slide
Journal: Colloids and Surfaces A: Physicochemical and Engineering Aspects - Volume 489, 20 January 2016, Pages 392–399