| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 69931 | 48802 | 2012 | 8 صفحه PDF | دانلود رایگان |
The α-glucosyl transfer enzyme (XgtA), a novel type α-glucosidase produced by Xanthomonas campestris WU-9701, was purified from the cell-free extract and characterized. The molecular weight of XgtA is estimated to be 57 kDa by SDS-PAGE and 60 kDa by gel filtration, indicating that XgtA is a monomeric enzyme. Kinetic properties of XgtA were determined for α-glucosyl transfer and maltose-hydrolyzing activities using maltose as the α-glucosyl donor, and if necessary, hydroquinone as the acceptor. The Vmax value for α-glucosyl transfer activity was 1.3 × 10−2 (mM/s); this value was 3.9-fold as much as that for maltose-hydrolyzing activity. XgtA neither produced maltooligosaccharides nor hydrolyzed sucrose. The gene encoding XgtA that contained a 1614-bp open reading frame was cloned, identified, and highly expressed in Escherichia coli JM109 as the host. Site-directed mutagenesis identified Asp201, Glu270, and Asp331 as the catalytic sites of XgtA, indicating that XgtA belongs to the glycoside hydrolase family 13.
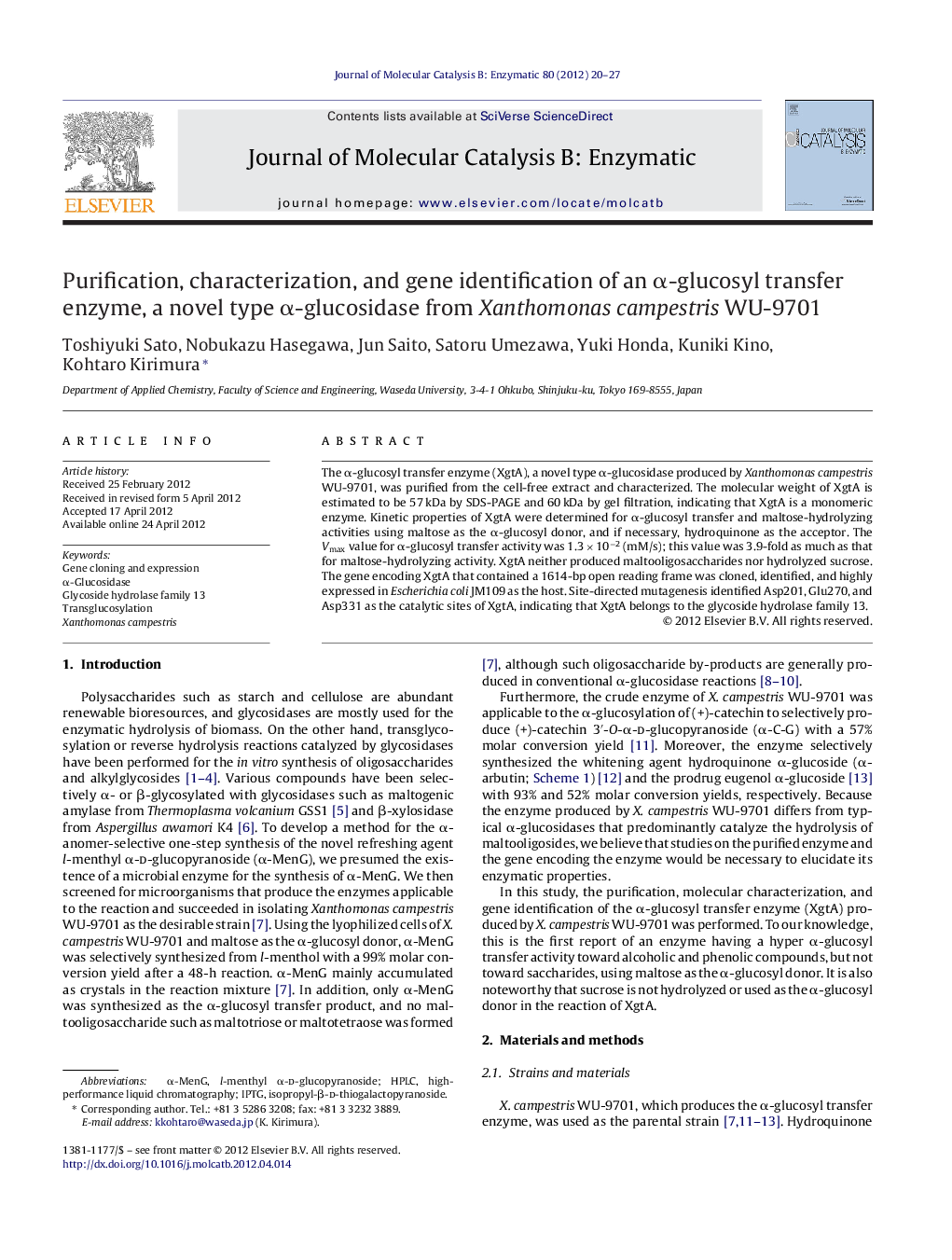
Three-dimensional modeling of the α-glucosyl transfer enzyme, a novel type α-glucosidase from Xanthomonas campestris WU-9701.Figure optionsDownload as PowerPoint slideHighlights
► The novel type α-glucosidase, XgtA, from Xanthomonas campestris WU-9701 was characterized.
► The α-glucosyl transfer activity of XgtA is higher than maltose-hydrolyzing activity.
► The gene encoding XgtA, a 1614-bp open reading frame, was identified.
► XgtA belongs to the glycoside hydrolase family 13.
Journal: Journal of Molecular Catalysis B: Enzymatic - Volume 80, August 2012, Pages 20–27