| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 217147 | 1426297 | 2009 | 8 صفحه PDF | دانلود رایگان |
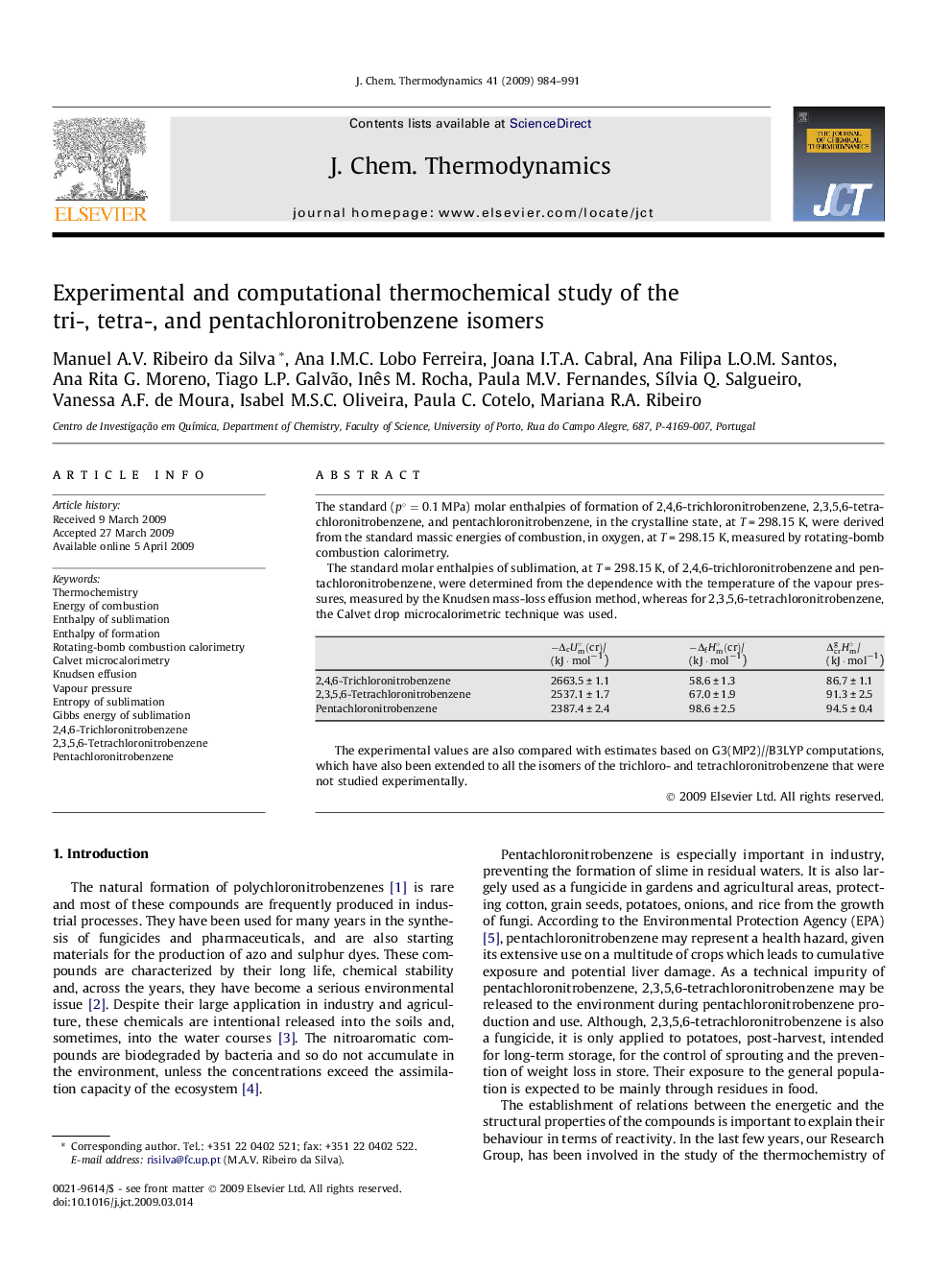
The standard (p∘=0.1MPa) molar enthalpies of formation of 2,4,6-trichloronitrobenzene, 2,3,5,6-tetrachloronitrobenzene, and pentachloronitrobenzene, in the crystalline state, at T = 298.15 K, were derived from the standard massic energies of combustion, in oxygen, at T = 298.15 K, measured by rotating-bomb combustion calorimetry.The standard molar enthalpies of sublimation, at T = 298.15 K, of 2,4,6-trichloronitrobenzene and pentachloronitrobenzene, were determined from the dependence with the temperature of the vapour pressures, measured by the Knudsen mass-loss effusion method, whereas for 2,3,5,6-tetrachloronitrobenzene, the Calvet drop microcalorimetric technique was used.-ΔcUm∘(cr)/(kJ · mol−1)-ΔfHm∘(cr)/(kJ · mol−1)ΔcrgHm∘/(kJ · mol−1)2,4,6-Trichloronitrobenzene2663.5±1.158.6±1.386.7±1.12,3,5,6-Tetrachloronitrobenzene2537.1±1.767.0±1.991.3±2.5Pentachloronitrobenzene2387.4±2.498.6±2.594.5±0.4Full-size tableTable optionsView in workspaceDownload as CSVThe experimental values are also compared with estimates based on G3(MP2)//B3LYP computations, which have also been extended to all the isomers of the trichloro- and tetrachloronitrobenzene that were not studied experimentally.
Journal: The Journal of Chemical Thermodynamics - Volume 41, Issue 9, September 2009, Pages 984–991