| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 593807 | 1453956 | 2013 | 12 صفحه PDF | دانلود رایگان |
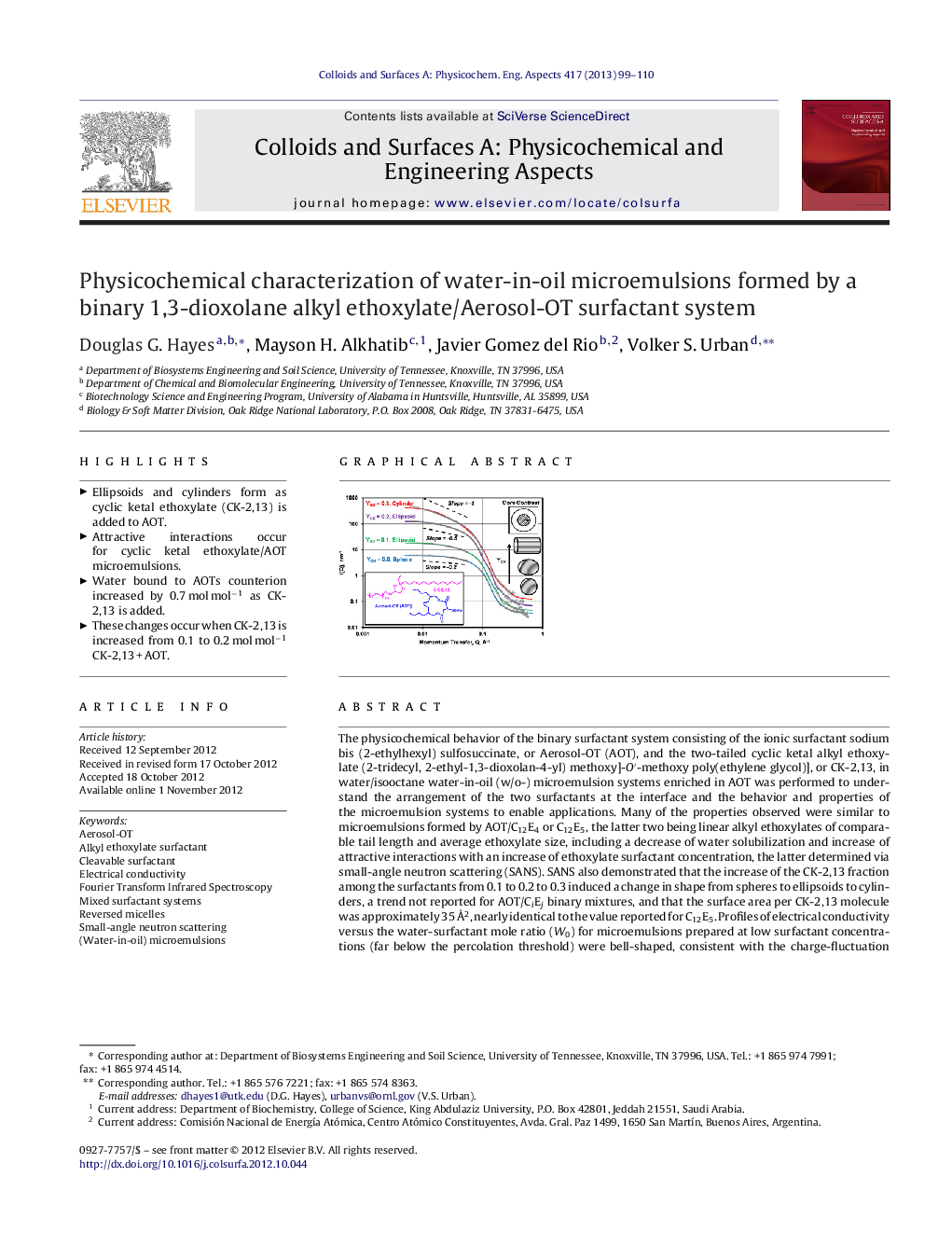
The physicochemical behavior of the binary surfactant system consisting of the ionic surfactant sodium bis (2-ethylhexyl) sulfosuccinate, or Aerosol-OT (AOT), and the two-tailed cyclic ketal alkyl ethoxylate (2-tridecyl, 2-ethyl-1,3-dioxolan-4-yl) methoxy]-O′-methoxy poly(ethylene glycol)], or CK-2,13, in water/isooctane water-in-oil (w/o-) microemulsion systems enriched in AOT was performed to understand the arrangement of the two surfactants at the interface and the behavior and properties of the microemulsion systems to enable applications. Many of the properties observed were similar to microemulsions formed by AOT/C12E4 or C12E5, the latter two being linear alkyl ethoxylates of comparable tail length and average ethoxylate size, including a decrease of water solubilization and increase of attractive interactions with an increase of ethoxylate surfactant concentration, the latter determined via small-angle neutron scattering (SANS). SANS also demonstrated that the increase of the CK-2,13 fraction among the surfactants from 0.1 to 0.2 to 0.3 induced a change in shape from spheres to ellipsoids to cylinders, a trend not reported for AOT/CiEj binary mixtures, and that the surface area per CK-2,13 molecule was approximately 35 Å2, nearly identical to the value reported for C12E5. Profiles of electrical conductivity versus the water-surfactant mole ratio (W0) for microemulsions prepared at low surfactant concentrations (far below the percolation threshold) were bell-shaped, consistent with the charge-fluctuation model, and shifted to lower W0 as the alkyl ethoxylate fraction was increased. The extent of the shift was greater for CK-2,13 than for an equal proportion of C12E4, suggesting CK-2,13 possesses a more profound influence on microemulsion properties. Analysis of the OH stretching region (3100–3700 cm−1) of the Fourier Transform Infrared Spectroscopic spectrum for w/o-microemulsions demonstrated that an increase of the CK-2,13 content of the total surfactant led to an increase of water molecules localized near the Na+ counterion of AOT of 0.7 mol water per mol surfactant, suggesting the water molecules of hydration for the ethoxylate group reside near AOT's counterion, resulting in increased dissociation of the counterion, hence to an increased hydrophilicity for AOT.
Figure optionsDownload as PowerPoint slideHighlights
► Ellipsoids and cylinders form as cyclic ketal ethoxylate (CK-2,13) is added to AOT.
► Attractive interactions occur for cyclic ketal ethoxylate/AOT microemulsions.
► Water bound to AOTs counterion increased by 0.7 mol mol−1 as CK-2,13 is added.
► These changes occur when CK-2,13 is increased from 0.1 to 0.2 mol mol−1 CK-2,13 + AOT.
Journal: Colloids and Surfaces A: Physicochemical and Engineering Aspects - Volume 417, 20 January 2013, Pages 99–110