| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 6451907 | 1416986 | 2017 | 8 صفحه PDF | دانلود رایگان |
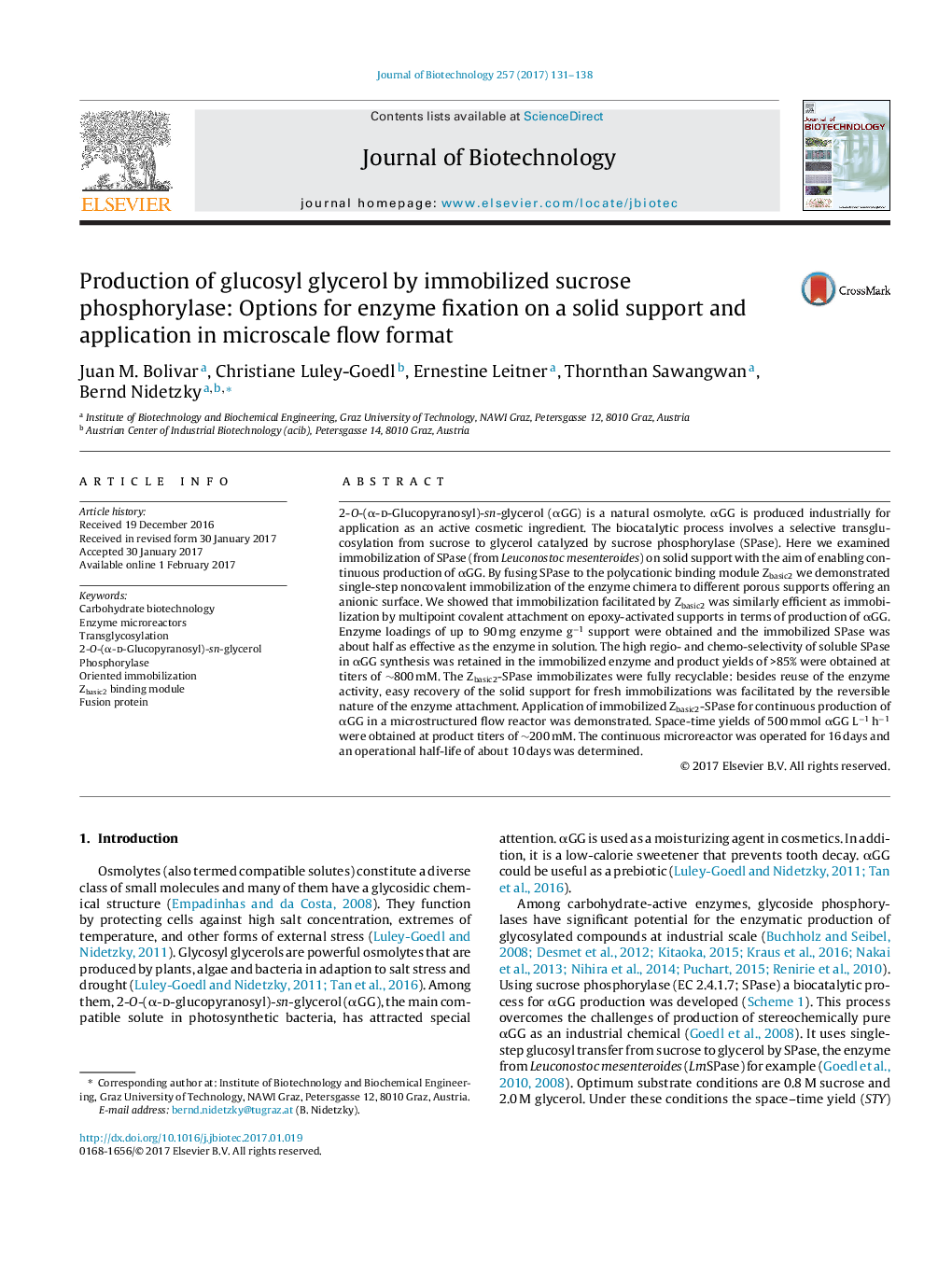
- Oriented non-covalent immobilization via Zbasic2 module compared to covalent immobilization.
- Enzyme immobilizates useful for production of α-glucosyl glycerol from sucrose and glycerol.
- Up to 90Â mg enzyme loaded on solid support to give highly active immobilizate.
- Product yields of 85% and product titers of 800Â mM reached at high reaction selectivity.
- High productivity (500Â mMÂ hâ1) obtained with immobilized enzyme in a microstructured flow reactor.
2-O-(α-d-Glucopyranosyl)-sn-glycerol (αGG) is a natural osmolyte. αGG is produced industrially for application as an active cosmetic ingredient. The biocatalytic process involves a selective transglucosylation from sucrose to glycerol catalyzed by sucrose phosphorylase (SPase). Here we examined immobilization of SPase (from Leuconostoc mesenteroides) on solid support with the aim of enabling continuous production of αGG. By fusing SPase to the polycationic binding module Zbasic2 we demonstrated single-step noncovalent immobilization of the enzyme chimera to different porous supports offering an anionic surface. We showed that immobilization facilitated by Zbasic2 was similarly efficient as immobilization by multipoint covalent attachment on epoxy-activated supports in terms of production of αGG. Enzyme loadings of up to 90 mg enzyme gâ1 support were obtained and the immobilized SPase was about half as effective as the enzyme in solution. The high regio- and chemo-selectivity of soluble SPase in αGG synthesis was retained in the immobilized enzyme and product yields of >85% were obtained at titers of â¼800 mM. The Zbasic2-SPase immobilizates were fully recyclable: besides reuse of the enzyme activity, easy recovery of the solid support for fresh immobilizations was facilitated by the reversible nature of the enzyme attachment. Application of immobilized Zbasic2-SPase for continuous production of αGG in a microstructured flow reactor was demonstrated. Space-time yields of 500 mmol αGG Lâ1 hâ1 were obtained at product titers of â¼200 mM. The continuous microreactor was operated for 16 days and an operational half-life of about 10 days was determined.
Journal: Journal of Biotechnology - Volume 257, 10 September 2017, Pages 131-138